/ CVal: THE INNOVATIVE SOLUTION FOR DIGITAL QUALIFICATION AND PROCESS VALIDATION IN THE REGULATED INDUSTRY
The CVal software developed by J&K Technology GmbH is the answer to the constantly increasing regulatory requirements in the regulated industry over the last few years.
With CVal you can efficiently implement your qualification and validation activities and ensure continuous traceability.
By using a single application for all qualification and validation activities, you avoid redundant data, interfaces and malfunctions. The error-proneness of your processes is automatically reduced and improved data quality is ensured.
Compare the efficiency and quality of the same plants or locations by using benchmarking functions and the processing of holistic key performance indicators (KPls).

/ INCREASED REQUIREMENTS FOR PHARMACEUTICAL COMPANIES
In particular, guidelines for quality assurance have been laid down in the GMP („Good Manufacturing Practice“) guideline – Annex 15: „Qualification and Validation“. The „Guidance for lndustry“ published by the FDA („Food and Drug Administration“, USA) confronts pharmaceutical companies with the ever-increasing task of meeting increasing requirements for process validation. Since then, mainly paper-based documents and data were to be adapted to the increasing and more complex requirements for plant qualification and process validation.
One consequence of these requirements is the need for electronic qualification and validation. The currently most common procedure for this is the creation and processing of data in various software systems and archiving by means of file systems. The consequences of these approaches are redundant and non-as-built data, increased susceptibility to errors due to the use of different systems and interfaces as well as a weak control system and time- and cost-intensive maintenance. This approach thus makes it difficult to ensure data integrity, quality, consistency and topicality.
/ THE SOLUTION: CVAL
Based on the Siemens COMOS software, CVal enables all qualification and validation processes to be carried out paperlessly in a single application. All relevant data can be checked and approved in the system and networked directly with the respective plant equipment.
CVal represents an independent solution in COMOS and enables the mapping of a digital Q&V twin. Through the virtual representation of the data, the plant and the process, it ensures a seamless object-based connection of the individual process steps. It offers the possibility to implement these processes by means of elaborated migration concepts and import/export functions and thus generates added value across departments. The CVal functionalities can be rolled out modularly.
Together with Siemens, J&K Technology GmbH thus offers an up-to-date software integration procedure that enables your company to create real structures, migrate data and qualify and validate your systems and processes object-based and digital in a central application. Benefit from our Q&V experience of the last 25 years, combined with the Engineering/Comos know-how of the last 10 years.
CVal combines project-specific modules and its specific system functions in only one application, enabling the implementation of common GMP requirements. In order to network the data perfectly, it is recommended to implement interfaces between Comos/CVal and SAP.
/ Project-specific Modules
Generation of a digital structure twin to be able to maintain and manage plant and location data.
The establishment of defined authorization levels is made possible by a simple implementation of the existing roles in the company.
Databases can be transferred using standard concepts. Individual documents/data package transfers can be carried out using import/export functions (e.g. Excel).
Creation and management of object-based reports is made possible by means of standard templates and version controls of the approved documents and reports.
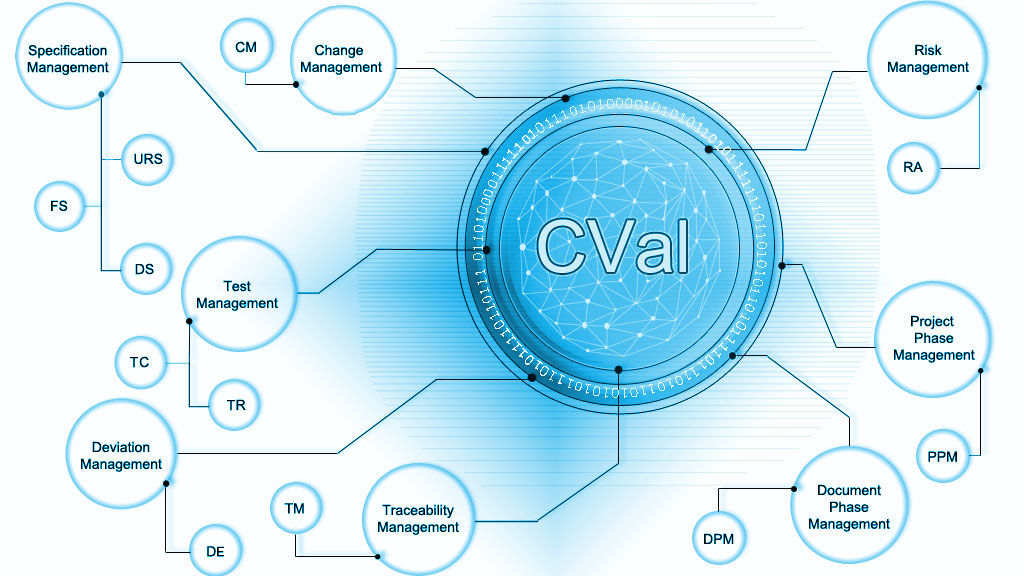
/ CVal-Modules
The specification management combines the documented specifications required in the official specifications, such as B. URS, FS and DS. Thanks to the object-based approach, these are available below plants, sub-plants, equipment and sub-equipment and enable clear identification and optimized life cycle management.
Risk management supports the identification, assessment, minimization, control and follow-up of risks in the various qualification and validation stages. The module enables the use of various and individual risk analysis methods (e.g. FRA, FMEA, QNA).
With this module, predefined test templates can be selected and the execution of the tests in the system documented.
Traceability Management enables you to link logically related objects (e.g. URS, RA, test case, deviation) in an up-to-date manner. An „as-built“ traceability matrix is only a by-product of the system. An initial impact analysis, for example, when a specification point is changed, is carried out in a very short time.
Deviation management records, evaluates, defines and controls a wide variety of deviation types and origins. Be it from a test phase or from a periodic review.
With the help of change management, changes to an existing system, process or plant are applied for, planned, evaluated, implemented and documented in a system-supported manner.
Document phase management allows all project-relevant and life cycle documents to be defined and scheduled. The transition between phases and the handover of the project documentation to the operator is only possible if previously defined documents including approval are available. The document information (e.g. phase assignment) can be edited, checking and approval activities are ensured by means of role assignment and e-mail notifications.
In project phase management, the various forms of the project and life cycle phases are documented in the system. This allows parallel processes to be better organized and dependencies in serial processes to be visualized. In addition, they are linked to the associated objects including data.
/ Contact
J&K Technology – Your digital GMP-Expert
J&K Technology GmbH
Kirchstrasse 11 | D-41569 Rommerskirchen
+49 2183 41894-0
